
Most anions are composed of nonmetals, which have high electronegativity. This is because anions have a higher electron affinity (tendency to gain electrons). A - sign mean the molecule has an overall negative charge, so it must have this extra electron. if NO 3 - has a negative charge of 1-, then you add 1 extra electron to the total 5 + 3(6)= 23 +1 = 24 total electrons).

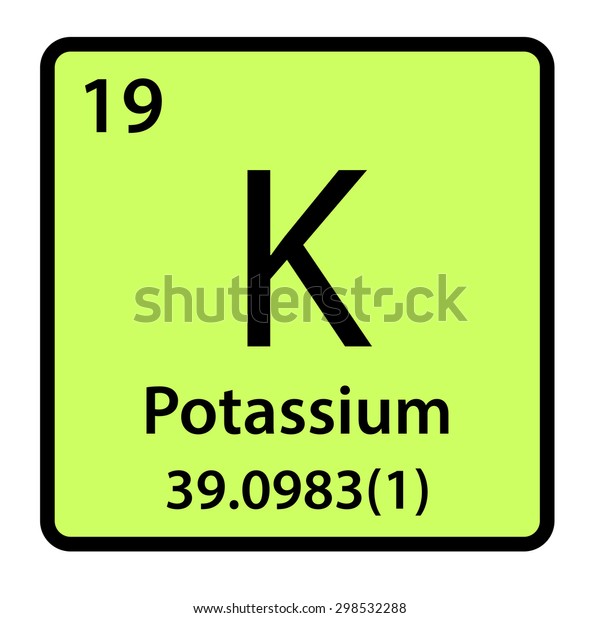
NOTE: There are TWO hydrogen atoms, so multiply 1 valance electron X 2 atomsĢ) If the molecule in question is an ion, remember to add or subract the respective number of electrons to the total from step 1.įor ions, if the ion has a negative charge (anion), add the corresponding number of electrons to the total number of electrons (i.e. Hydrogen (H)-Group IA: therefore, there is 1 valance electron Oxygen (O)-Group VIA: therefore, there are 6 valance electrons Follow the roman numeral group number to see the corresponding number of valance electrons there are for that element. Remember, if there are two or more of the same element, then you have to double or multiply by however many atoms there are of the number of valance electrons. To draw the lewis diagrams for molecular compounds or ions, follow these steps below (we will be using H 2O as an example to follow):ġ) Count the number of valance electrons of the molecular compound or ion. Lewis diagrams for Molecular Compounds/Ions The nonindicated transition metals, lanthanoids, and actinoids are more difficult in terms of distinguishing the number of valance electrons they have however, this section only introduces bonding, hence they will not be covered in this unit. The alkali metals of Group IA have one valance electron, the alkaline-earth metals of Group IIA have 2 valance electrons, Group IIIA has 3 valance electrons, and so on. Generally the Roman numeral of the group corresponds with the number of valance electrons of the element.īelow is the periodic table representation of the number of valance electrons.

However, the general convention is to start from 12o'clock position and go clockwise direction to 3 o'clock, 6 o'clock, 9 o'clock, and back to 12 o'clock positions respectively. The position of the valence electrons drawn is unimportant. In a Lewis diagram of an element, the symbol of the element is written in the center and the valence electrons are drawn around it as dots. Valance electrons are the electrons that form the outermost shell of an atom. Lewis diagrams are graphical representations of elements and their valence electrons. For each set of elements, state one characteristic that they have in common.\) Science Quest 10 Student Workbook © John Wiley & Sons Australia, Ltdģ. In each column arrange the group members in Sort these elements into their correct groups. The following 12 elements belong to four different groups of the periodic table.Įlements: potassium neon sulfur fluorine tellurium argon lithium oxygen iodine Classify the elements labelled A–F as metals, non-metals or semi-metals.Ī. The following diagram illustrates the first four periods of the periodic table. Elements can be classified into groups called metals, non-metals and semi-metals (or © John Wiley & Sons Australia, Ltd Science Quest 10 Student Workbookġ. Lithium Oxygen Fluorine Helium Potassium Sulfur Bromine Neon Rubidium Tellurium Iodine Argon Worksheet 4.1 Science Quest 10: pages 166 – 73 Non-metal Non-metal Metal Metal Semi-metal (metalloid) Non-metal In each column arrange the group members in order of increasing atomic weight. Elements: potassium neon sulfur fluorine tellurium argon lithium oxygen iodine rubidium helium bromine Sort these elements into their correct groups. The following 12 elements belong to four different groups of the periodic table. Classify the elements labelled A – F as metals, non-metals or semi-metals. Elements can be classified into groups called metals, non-metals and semi-metals (or metalloids). © John Wiley & Sons Australia, Ltd Science Quest 10 Student Workbook CHAPTER 4: Chemical patterns Periodic table Answers Classifying the elements 1.


 0 kommentar(er)
0 kommentar(er)
